Think about the last time you took medicine, ate packaged food, or used a skincare product. You probably trusted that it was safe to use. But have you ever wondered how companies make sure these products will not harm you? That is where GxP compliance matters.

Whether you are running a pharmaceutical company, food factory, or a biotech startup, understanding and following GxP can make or break your success. In this blog, we will walk you through:
- What is GxP compliance?
- Why is it so important?
- What happens if companies ignore it?
- And how following it can benefit your business and customers.
Even though the term “GxP” might sound complicated, it is something that helps protect people, ensure product quality, and build trust in businesses. Let us get started.
What Is GxP Compliance: Everything You Need to Know
GxP is a general term that stands for “Good x Practice”, where “x” is a placeholder for different types of industries and activities. The “G” stands for Good, the “P” stands for Practice, and the “x” can be replaced with things like:
- GMP =Good Manufacturing Practice
- GLP = Good Laboratory Practice
- GCP = Good Clinical Practice
- GDP = Good Distribution Practice
- GVP = Good Pharmacovigilance Practice
All of these practices are part of what’s called GxP guidelines. They exist to make sure that products like medicines, food, cosmetics, and medical devices are,
- Safe to use.
- Made correctly.
- Properly tested.
- Stored and transported the right way.
These guidelines are followed globally by different companies.
Why Is GxP Compliance Important?
GxP compliance refers to a set of quality guidelines and regulations followed in industries like pharmaceuticals, biotechnology, and food production to ensure products are safe, effective, and meet quality standards. Understanding its importance helps us see how these regulations protect not just businesses, but also public health and safety.
1. Protecting Human Health
First and foremost, GxP is all about safety. Imagine what could happen if a medicine were not tested properly or a food product were made in an unclean facility. People could get sick or worse. GxP ensures that all steps in the development, production, and delivery of products are done in a clean, accurate, and traceable way.
For example, GMP ensures that drugs are consistently produced and controlled to the highest quality standards. According to the World Health Organization (WHO), poor-quality medicines are responsible for tens of thousands of preventable deaths every year.
2. Avoiding Legal and Financial Trouble
In most countries, GxP compliance is not optional. It is a legal requirement. Agencies like the FDA (U.S. Food and Drug Administration) e EMA (European Medicines Agency) regularly inspect businesses to ensure they follow GxP rules. If a company fails an inspection, it can face:
- Large fines
- Lawsuits
- Product recalls
- Shutdowns
A real-life example is Ranbaxy Laboratories, an Indian pharmaceutical company that had to pay $500 million in 2013 for not following GMP guidelines. This was one of the largest healthcare fraud settlements ever.
3. Maintaining Trust and Reputation
Consumers trust brands that follow the proper regulations and laws while producing products. If a company is known for quality and safety, customers are more likely to buy from it again. On the other hand, just one safety incident can permanently damage a brand’s reputation. In today’s digital age, news spreads fast, and one bad headline can lead to a long-lasting loss of customer confidence. So, creating the authenticity and assuring consumers about the product is important.
4. Ensuring Global Market Access
If you want to sell your products in international markets, you need to meet GxP standards. Many countries require proof of compliance before allowing imports. Without GxP certification or documentation, your product may be blocked at borders, causing business delays and financial losses.
What Does GxP Cover?
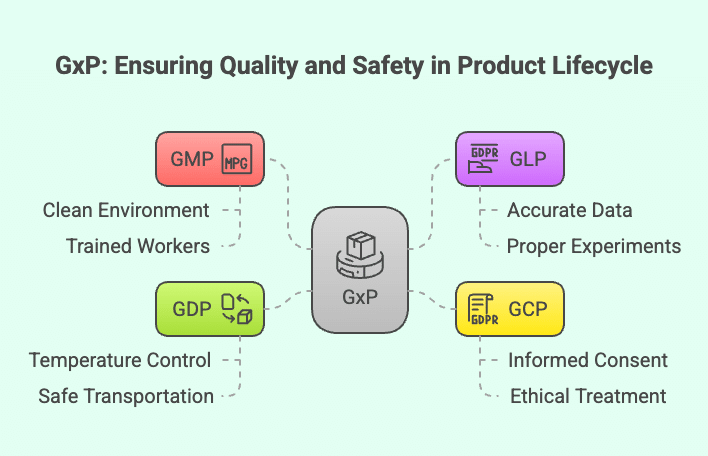
To understand why GxP is important, we need to look at the different types of standards, each focusing on a specific part of a product’s journey—from manufacturing and lab testing to clinical trials and final delivery. These standards ensure safety, quality, and reliability across multiple industries. Let us break it down:
🏭 Good Manufacturing Practice (GMP)
GMP focuses on how products are made, particularly ensuring they are produced in clean, controlled, and safe environments. This includes proper facility hygiene, well-maintained equipment, and trained personnel. GMP is not just for pharmaceuticals, it is also vital in food and beverage, cosmetics, medical devices, dietary supplements, and veterinary products. This applies not only to physical goods but also to software and digital products, which must be developed and managed under similar quality and safety standards.
Example: A food processing plant must follow GMP to avoid contamination and ensure product consistency. A single lapse could lead to foodborne illness outbreaks or massive recalls.
🔬 Good Laboratory Practice (GLP)
GLP ensures that laboratory testing is conducted with accuracy, transparency, and integrity. It covers proper experiment design, reliable data recording, and routine equipment calibration. While commonly used in pharmaceuticals, GLP is also essential in agriculture (e.g., pesticide testing), environmental sciences, chemicals, and cosmetic safety studies.
GLP is not just for physical lab testing, it also applies to software and digital tools. Whether it is a data analysis platform, a digital lab notebook, a plugin, or a cloud-based solution, the software must undergo validation, version control, and secure data handling, all core parts of GLP.
Example: In agriculture, GLP is used to test pesticide residues in crops to ensure they are safe for consumption. Without these standards, contaminated produce could reach consumers.
👨⚕️ Good Clinical Practice (GCP)
GCP comes into play when products, especially drugs or medical treatments, are tested on people. It ensures clinical trials are ethical and participants are protected. GCP is critical in the biotech, pharmaceutical, and medical device industries and is also increasingly applied in digital health and diagnostics.
Example: A wearable health-tech company conducting trials for a new glucose monitoring device must comply with GCP to ensure test results are credible and user safety is prioritized.
📦 Good Distribution Practice (GDP)
GDP makes sure products, especially temperature-sensitive ones like vaccines or biologics, are stored and transported under proper conditions. It is widely used in pharmaceuticals, medical devices, biologics, food and beverage logistics, and even specialty chemicals.
Example: During global COVID-19 vaccine distribution, GDP ensured that vaccines requiring cold-chain logistics maintained their efficacy from manufacturing sites to rural health centers.
Is GxP Compliance Required for a WordPress Company?
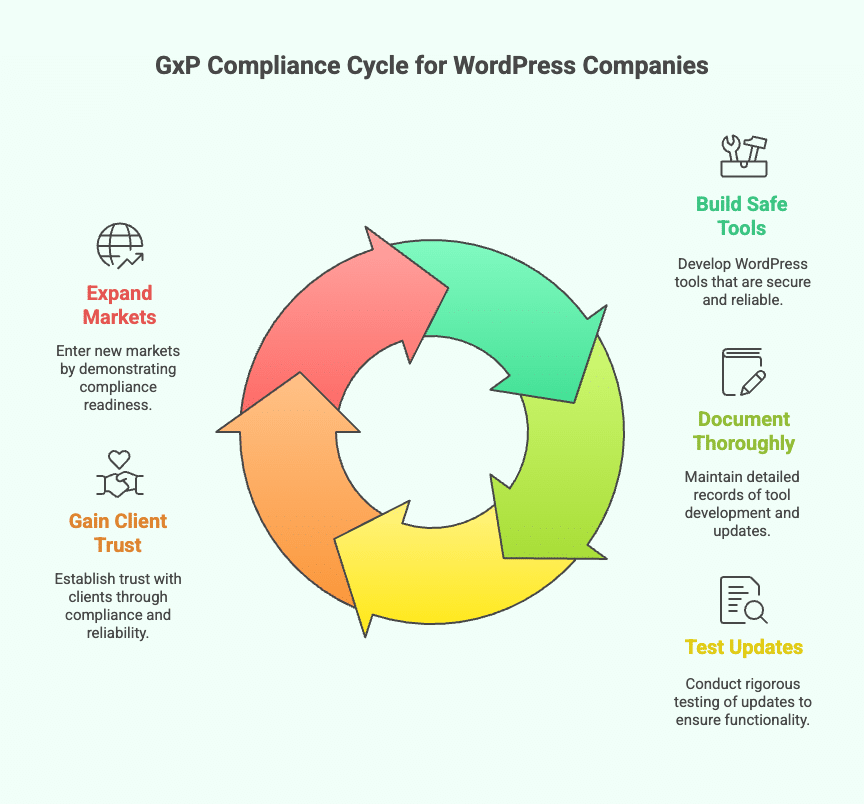
Some WordPress companies build themes, plugins, and full websites for businesses in fields like healthcare, medicine, and science. These businesses must follow strict rules called GxP (Good Practice) to make sure their data is correct, safe, and trustworthy. That is why WordPress companies need to follow GxP, too. Here is why it matters.
1. Clients Work in Legal Bound Industries
Many clients work in areas like healthcare and life sciences, where the rules are very strict. If they use WordPress tools to share things like clinical trial results or patient information, those tools must be safe, reliable, and well-documented to meet the rules they have to follow.
2. Protecting Data Is a Must
GxP rules say that businesses must keep accurate and secure records. That means WordPress tools like forms or plugins must be built carefully. A small mistake in a form, like collecting incomplete or incorrect patient data, could lead to compliance violations, legal penalties, or even risks to patient safety. So, everything has to be handled safely and clearly.
3. Updates Must Be Checked
Whenever the company updates a plugin or theme, clients in regulated industries might need to test it again to make sure it still works properly. That is why it is important to keep clear records of changes and follow good update practices. It helps clients trust the product.
4. Understanding GxP Builds Trust
Companies in healthcare and similar fields want to work with partners who understand their needs. When a WordPress company shows that it knows about GxP and builds tools with compliance in mind, it becomes a trusted partner. This can lead to stronger, long-term business relationships.
5. Prepares for the Future
More and more industries are starting to use WordPress. If a WordPress company builds its products with care, quality, and rules like GxP in mind now, they become careful about more rules that might come ahead.
Benefits of GxP Compliance for Tech Companies
For tech companies, following GxP (Good Practice) guidelines goes beyond simply avoiding issues. It lays the foundation for long-term growth and resilience. Here is how GxP compliance can make a significant difference:
✅ Better Software Quality
GxP guidelines promote careful and consistent development practices, resulting in fewer bugs, smoother performance, and greater user satisfaction. Clients and users are more likely to trust tools that function reliably and meet high standards.
✅ Increased Efficiency
GxP emphasizes clear documentation and structured workflows. This reduces confusion, accelerates development timelines, and helps teams release updates on schedule. Everyone involved knows their role and how to execute tasks effectively.
✅ Fewer Errors and Lower Legal Risk
Mistakes, especially in software used by clients in healthcare or life sciences, can be costly or even dangerous. GxP helps tech companies or software companies create safer, more reliable products while minimizing legal risks and avoiding customer disputes.
✅ Faster Client Approvals
Clients often require regulatory approvals for their digital tools. When software is developed in line with GxP, it supports quicker, smoother approvals from health and government agencies, enhancing the tech provider’s value as a partner.
✅ Access to New Markets
Many international markets have strict regulatory requirements for software in regulated industries. GxP-compliant products can more easily enter and thrive in these markets, unlocking broader growth opportunities.
✅ Enhanced Brand Trust
A reputation for following best practices in development and compliance boosts brand credibility. Companies known for GxP adherence are seen as reliable, professional, and safe, making them stand out in competitive landscapes.
A 2020 Deloitte report revealed that companies with robust compliance programs experienced up to 25% faster time-to-market for new products and reduced costs related to rework and recalls.
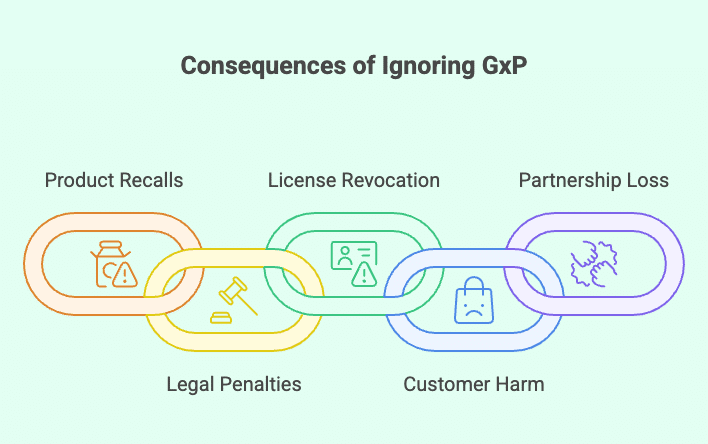
What Happens If a Business Ignores GxP?
Following GxP is not just about ticking off checklists. GxP compliance is about protecting people and building trust. This helps keep a business running smoothly. When companies ignore these important rules, the consequences can be serious, not just for the business but also for customers and the public.
A study by McKinsey found that compliance failures can reduce a company’s value by 10–15% or more, depending on how severe the issue is. Here is a closer look at what can go wrong when businesses do not follow GxP guidelines:
🚫 Product Recalls
If a product is found to be unsafe, poorly made, or incorrectly labeled, it may have to be removed from stores and returned; this is called a recall. Recalls are expensive because companies lose all the money spent making and shipping the product. They also take a lot of time and effort to fix. On top of that, recalls can damage a company’s image, making customers less likely to trust their products in the future.
⚖️ Fines and Legal Action
Governments and regulatory organizations take GxP very seriously. If a company breaks the rules, it can face legal consequences, including lawsuits or large fines. These penalties can cost millions of dollars. In more serious cases, company leaders might even face criminal charges, especially if people’s health or safety was put at risk.
📉 Loss of Certification or License
Businesses that make medicines, food, or medical devices often need a license or certification to legally operate. If they don’t follow GxP standards, that license can be taken away. This means the company may have to stop making or selling its products altogether until it fixes the problem, and sometimes, it may not get the license back at all.
👎 Injured or Unhappy Customers
One of the most important reasons to follow GxP is to protect customers. If a company ignores safety rules, the products it sells could hurt people. For example, a contaminated drug or spoiled food could make someone seriously ill. Similarly, the software that they are using can harm their device or put their website at risk. Even if no one is physically harmed, poor-quality products can lead to complaints, refunds, bad reviews, and a loss of trust in the brand.
⚠️ Loss of Business Partners
Companies do not work alone. They depend on suppliers, distributors, and retailers to help get their products to customers. If a business is known for not following GxP, other companies may stop working with them because they do not want to be associated with poor practices. This can hurt future opportunities and limit growth.
Real-Life Examples of GxP Compliance Impact
GxP compliance is not just something companies do to follow rules, it can be the reason a business succeeds or fails. Real-world cases help highlight how adherence to these regulations has had major consequences for companies and their consumers.
✅ Success Story: Pfizer and the COVID-19 Vaccine
Pfizer, one of the largest pharmaceutical companies in the world, strictly follows GxP guidelines. During the COVID-19 pandemic, their ability to develop, test, and distribute a safe vaccine quickly was possible because of their existing GxP-compliant systems. The vaccine passed global health checks and reached millions of people faster, saving countless lives.
📍 Learning from Challenges: Valeant Pharmaceuticals
Valeant Pharmaceuticals (now Bausch Health) faced challenges related to quality control and regulatory compliance. These issues led to investigations and warnings from health authorities. The situation highlighted how important it is for companies to consistently follow GxP standards to maintain trust, ensure product safety, and protect their long-term reputation.
How Can Businesses Stay GxP Compliant?
Staying GxP compliant might seem complicated at first, but it becomes manageable with the right approach. Following a few key steps helps businesses build a strong, reliable system that meets all the rules and keeps quality high.
1. Employee Training: Everyone involved, from lab workers to factory staff, must be trained regularly. Understanding why and how to follow GxP rules is key.
2. Clear Documentation: Proper records are the backbone of GxP. This includes batch records, test results, quality checks, and more. If something goes wrong, good documentation shows where and why.
3. Internal and External Audits: Regular audits help catch problems early. Internal audits ensure your team is following procedures, and external audits by regulators give you feedback to improve.
4. Use Digital Tools and Software: Modern businesses use Quality Management Systems (QMS) to track everything from employee training to product testing. Tools like MasterControl, Veeva, and TrackWise help automate compliance and reduce errors.
GxP Compliance: A Commitment to Quality, Safety & Trust
GxP compliance might sound like just a set of rules, but it is much more than that. It is about protecting people, building trust, ensuring quality, and helping businesses grow. For companies in regulated industries like pharma, biotech, food, and medical devices, following GxP is not just a best practice; it is a necessity.
Whether you are running a company or just curious about how the products you use are made safe, understanding GxP is important. A GxP-compliant business is a responsible business—one that values both quality and people’s lives.
Was this blog helpful to you? Feel free to share your thoughts in our Facebook Community. Also, subscribe to our blogs to get more blogs like this.